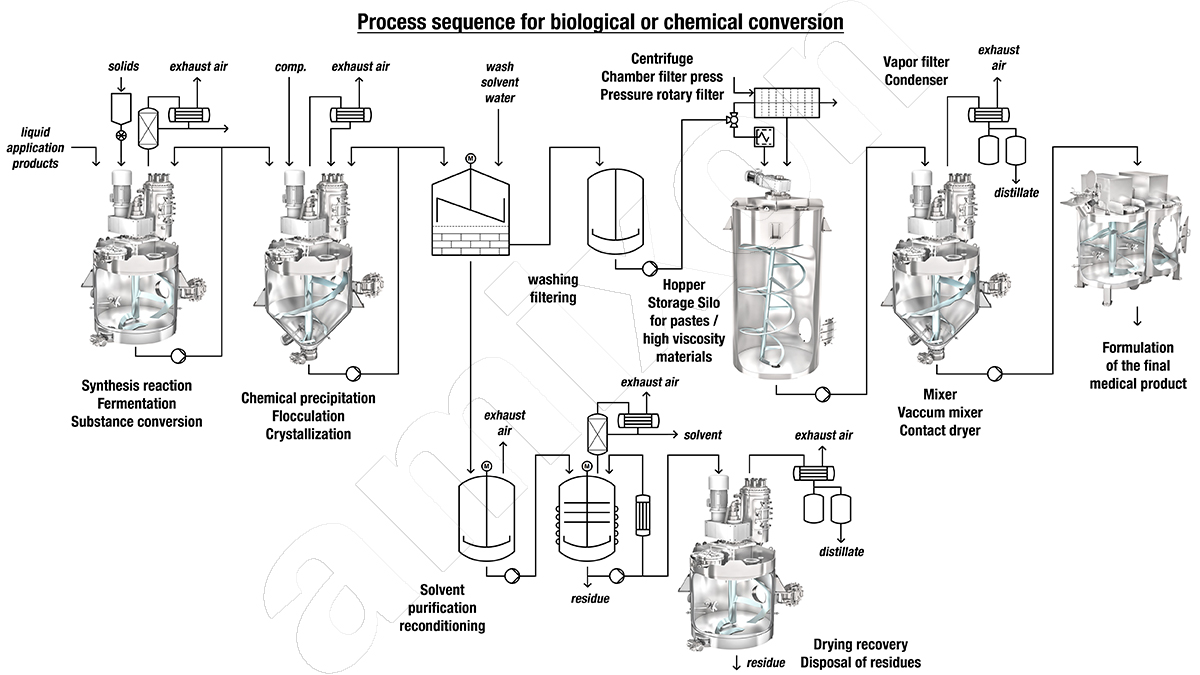
Which plant is best suited for the production of APIs (active pharmaceutical ingredients)?
In the production of pharmaceutical ingredients, the efficiency of the mixing technology is of crucial importance, regardless of whether the reactions are biotechnological or chemical. The ultimate goal is complete chemical/biochemical conversion.
Only when the entire reactor content is completely flowed through/mixed can the substances involved in the reaction come into contact with each other, uniformly distributed. Only then can a uniform temperature level be achieved in the reactor. Process temperatures can only be reliably controlled in this way.
Yield, quality and production safety depend directly on the efficiency of the mixing process.
Quality-determining apparatus properties:
- It is important that the mixer synthesis reactor works gently.
- The mixing tools may only move slowly.
- Nevertheless, the mixing quality must always be ideal, regardless of whether it is a liquid, a suspension or a highly viscous mass.
- The resulting suspensions, emulsions, crystals, coagulates, aggregates or flakes must not be sheared or damaged.
In order to be able to control the thermokinetic processes reliably, the synthesis reactor/fermenter must have heat exchanger surfaces that are as large as possible. The heat exchanger surfaces must have the same temperatures at all points. Steam, water or thermal oil can be used as heat transfer media. The flow resistance in the heat transfer walls must be kept to a minimum. amixon® has invested heavily in research and development in this area. amixon® can offer various solutions depending on the desired batch size. amixon® can demonstrate the respective efficiencies in its own technical centre.
Biofermenters/ bioreactors/ mixers/ fumigation system for API production
In this case, the starting materials are usually in the form of suspensions or lipophilic emulsions. For biotechnological conversions, the even distribution of oxygen, nutrients, enzyme catalysts and nutrient solutions is crucial. For a homogeneous growth of the microorganisms, the temperature must be controlled to within a tenth of a degree. The cell growth of the microorganisms in the nutrient solution leads to altered flow properties. Anchor stirrers or propellers are unsuitable as stirring elements when the viscosity increases.
SinConvex® / SinConcave® spiral mixing elements produce ideal mixing results even when the consistency changes (comparable to honey or molasses). They are particularly gentle and always efficient. Low speeds are particularly important. This means low friction, low pressure and low shear stress. It is important for fermentation to avoid heat input caused by friction.
Gaseous inputs
In most cases, oxygen is blown into the reactor in the form of sterile air. This promotes the growth of aerobic microorganisms. However, there are also cases in which carbon dioxide (CO2) or gaseous ammonium (NH3) are introduced to regulate the pH. The introduction of hydrogen (H2) can change the energy Nitrogen (N2) is used for inertisation, especially when microorganisms only grow under anaerobic conditions.
The more finely the gas is introduced, the better the efficiency. The density of the reactor content is reduced. This decreases the flow resistance and the mechanical shear stress when mixing the masses. Sensitive cell cultures are better protected against shear and pressure.
Bid selection by means of utility analysis
There are a large number of manufacturers of synthesis reactors and fermenters. Many apparatus manufacturers have specialised in certain process steps or end products. When an investor is planning a modern plant, it is not easy to find the most suitable apparatus manufacturer.
In addition to many other aspects, fundamental requirements must be met:
- precise reaction control,
- process efficiency (yield and residues)
- universal applicability,
- hygiene and sterility,
- scalability,
- material resistance,
- automatic process monitoring,
- GMP compliance,
- flexibility,
- ergonomics and occupational safety,
- sustainability,.....
The attached table (PDF format) shows an example of how such an analysis can be carried out.
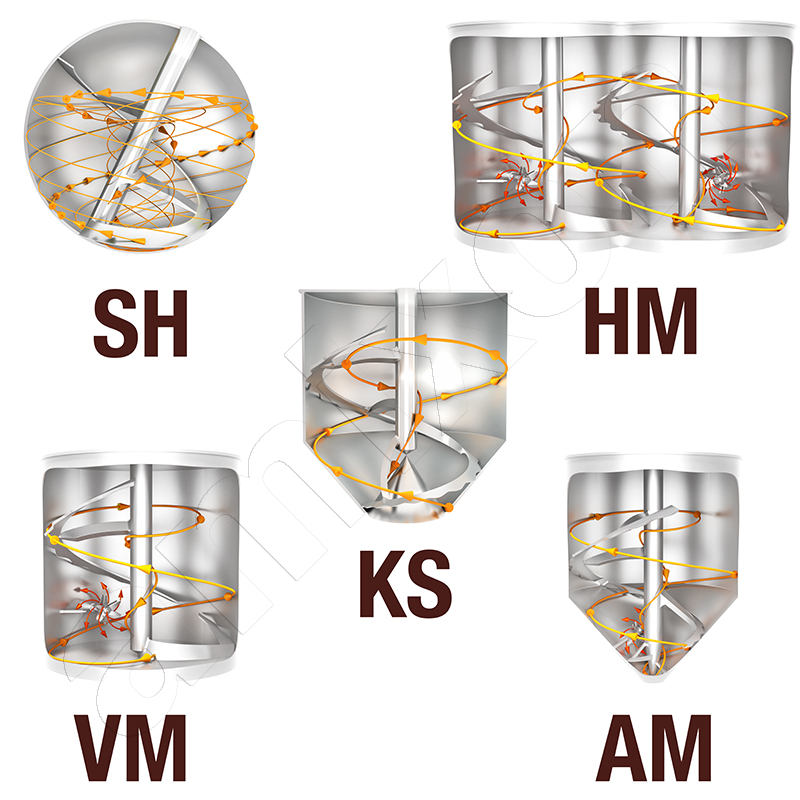
amixon® vertical precision mixers
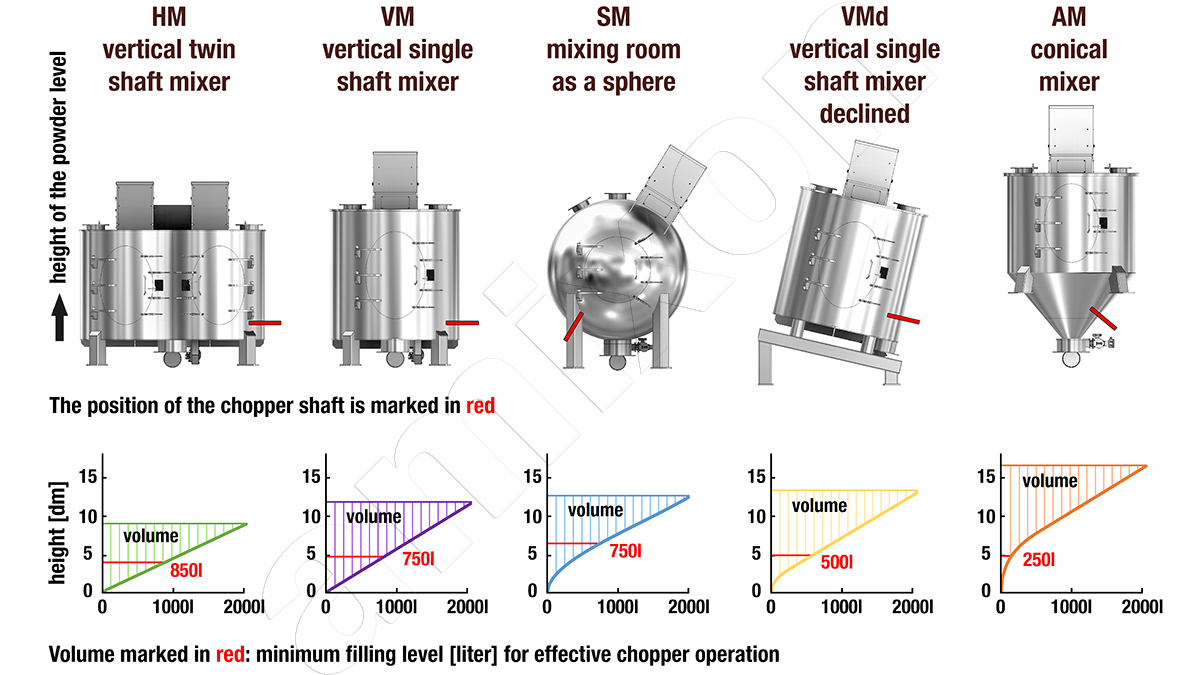
The red line shows the position of the shaft when a high-speed vortex tool is installed. This can be particularly effective for deagglomeration in addition to the main mixing tool.
Determining the optimal size of equipment
This question seems simple at first glance. In fact, it can be difficult to answer correctly.
In most cases, the bulk density remains constant when different goods (wet or dry) are poured together and mixed. However, the density can change significantly as a result of the reaction process. If the density increases, the filling level in the apparatus decreases. In this case, it must be ensured that the reactor/mixer/vacuum dryer/fermenter/bioreactor can also mix smaller volumes well and efficiently.
As density decreases, the volume of the mass increases. The apparatus must be large enough to accommodate and mix the correspondingly increased volume. Such volume changes cannot be calculated. If volume changes occur, they can only be determined by testing.
In contrast to liquid containers, mixers/reactors/vacuum dryers/fermenters/bioreactors must not be filled completely. They require a free space (gas space) above their filling level. This is necessary to enable the mixture to flow/swirl. The free space volume is the difference between the gross and net volume. The net volume is the maximum permissible filling level at which the mixing processes can still take place unhindered.
The manufacturers of mixers/reactors/vacuum dryers/fermenters/bioreactors define the free spaces (= gas volumes) of their devices differently depending on the design and mode of operation. If a cost-utility analysis is to be carried out, it is necessary to do so on the basis of the same net volumes. The design and production of amixon is very flexible. We can approximate the required net volume in 100-litre increments. amixon® defines the size designation based on the net volume = useful volume. If the type designation of an amixon® conical mixer/reactor is, for example, AMT 30400, the mixture inside it can have a volume of 30.4 m³. The gross volume of the amixon® apparatus is then approx. 40 m³.
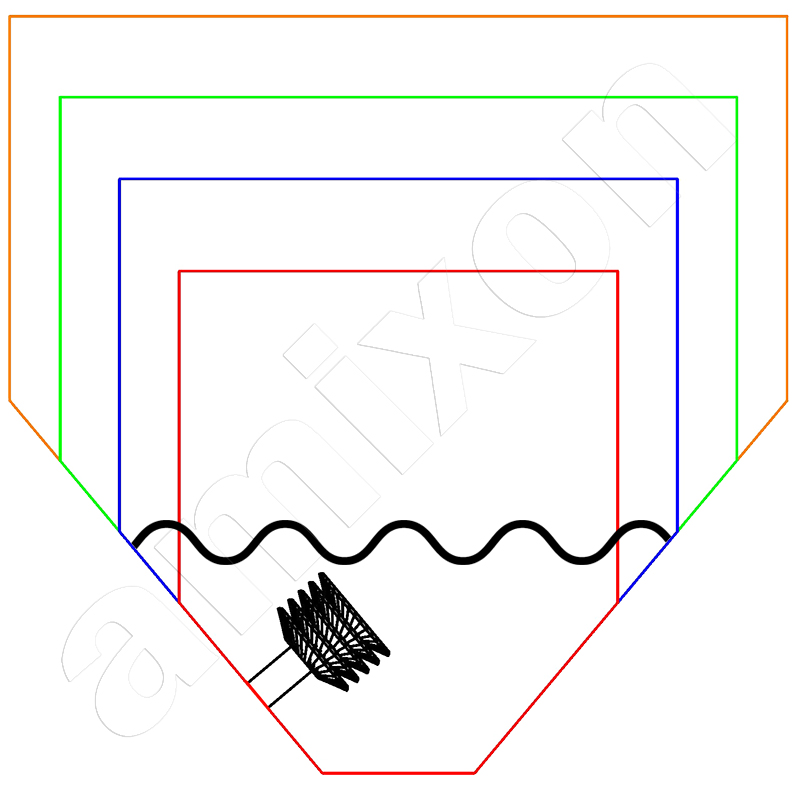

Biotechnological synthesis of active pharmaceutical ingredients (APIs) is less common than chemical synthesis
Nevertheless, biochemical processes will become more important in the future. This is due to three facts:
- Mild reaction conditions mean high energy efficiency and cost-effective apparatus and plant construction. In turn, a germ-free production environment must be ensured.
- Enzymes are biodegradable
- Unwanted by-products are usually less hazardous and can be disposed of cheaply. They are normally far less hazardous than reaction residues from chemical production.
Well-known pharmaceutical active ingredients produced using biological processes include insulin, erythrocytes, monoclonal antibodies, growth hormones, antibiotics, cholesterol-lowering drugs, vitamins (B12 and C), heparin and many vaccines against COVID. The more complex the molecular structures are, the more likely it is that so-called biotechnological production processes will be used. This is the case, for example, in personalised medicine.
Little research has been done on bio-similar microbial growth at boiling temperatures under high pressure and in a sulfuric acid environment (hot springs in the deep sea). Catalysts are also suspected here, but little is known about them.
Semisynthetic APIs
Some active pharmaceutical ingredients, such as antibiotics, semisynthetic opiates or steroid hormones, are produced by a combination of biological and chemical processes. The biological process usually takes place first. This method allows complex molecular structures to be built efficiently. Further chemical processing can improve purity and bioavailability by using organic solvents (polar and non-polar). There are also semi-synthetic drugs that are composed of synthetic and biological active ingredients.
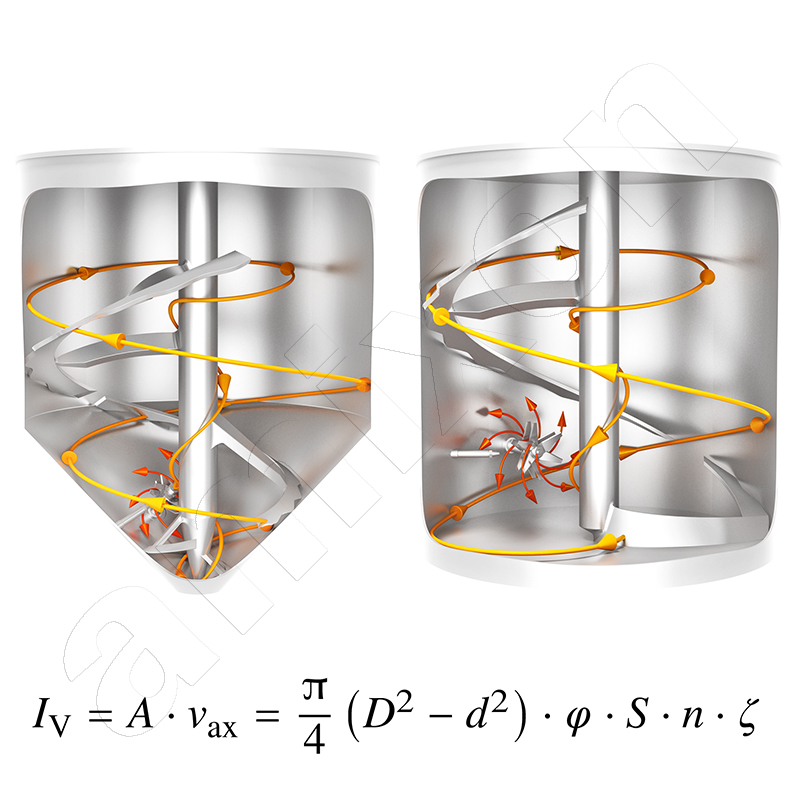
Three-dimensional mixing effect with no dead space
The mixing effect is generated by the spiral mixing tool. The materials to be mixed are conveyed from bottom to top. The conveyed volume flow Iv is calculated as follows.
Iv: volume flow conveyed from bottom to top by a spiral mixing tool
φ: is the filling level
D/ d: outer/inner diameter of the mixing helix
S: pitch of the helix
n: rotation frequency
ζ: speed coefficient
There is no need to worry about the downward flow in the mixing chamber. It is caused by gravity. Gravity acts equally on all particles. The upward and downward flows interact and cause a random change in the location of the particles / molecules / aggregates / agglomerates / coagulates /..... The entire process is overlaid by rotation.
As with all devices with rotating internals, the relative movement between the mixing tool and the mixture is crucial. The rotation alone does not produce any relative movement. The more the mixture is conveyed upwards by the mixing tool, the more effective the mixing action. In this respect, a vertical mixer can also produce optimum mixing qualities at very low speeds. All volume fractions in the mixing container are included and incorporated into the flow process, with no dead space.
amixon® supports your customers from the outset...
and, if desired, until successful commissioning. It is often difficult for manufacturers of active ingredients to find the optimal synthesis reactor and the right manufacturer. amixon GmbH provides support with a large technical centre and numerous pilot plants - a valuable aid in selecting the right system.
- First-class test results in the technical centre are the most important prerequisite for a purchase decision.
- Solid, well-engineered machine construction with a high level of in-house production guarantees reliability, spare parts supply and a long service life.
- Service calls and training, which amixon® has been providing worldwide for almost 40 years, enable the operator to benefit from cost advantages and stable value retention.
- The constant further development of amixon® products enables the retrofitting of older amixon® machines.
- amixon® employees with many years of experience are your competent points of contact. We stand for short response times and maximum efficiency.
- A high degree of transparency guarantees an optimal price-performance ratio.
In addition, amixon helps to meet important industry standards with established solutions:
- ATEX (ATmosphères Explosives) European legislation to prevent fires and explosions.
- OSHA (Occupational Safety and Health Administration; US legislation on occupational safety)
- FDA (Food and Drug Administration)
- GMP compliance (Good Manufacturing Practice)
- Support for full validation (Design Qualification, Installation Qualification, Operational Qualification, Performance Qualification)
- Internationally recognised automation standards
Enzymes are reaction accelerators
Enzymes consist of long amino acid chains. Catalysts lower the energy barrier that has to be overcome for a chemical reaction to take place. They can accelerate chemical conversion processes by a hundredfold.
If an enzyme accelerates chemical reactions without being consumed in the process, it is referred to as an enzyme catalyst. They accelerate biochemical reactions and act in a highly selective manner. They only act on certain molecules or groups of molecules.
- Hydrolases can break down ester bonds. They can also break down fats into glycerol and fatty acids.
- Oxidoreductases can accelerate redox reactions by splitting off hydrogen atoms or incorporating oxygen atoms into another molecule group.
- Transferases can split off methyl groups from molecules and incorporate them into foreign acceptors.
- Lyases are enzyme catalysts that work without the presence of water. They break down carbon-carbon, carbon-hydrogen or carbon-nitrogen bonds. Conversely, they can build double bonds in amino acids.
Enzymatic conversions appear unspectacular. They usually take place at moderate temperatures. The pH of the nutrient solutions is relatively neutral. Atmospheric conditions usually prevail.

Synthesis reactor/mixer for chemical syntheses
In the chemical synthesis of APIs, the reaction partners (such as reagents, solvents and catalysts) must be uniformly and randomly distributed at all times. Only in this way can an optimal reaction rate and yield be achieved.
In the production of powdery/crystalline active pharmaceutical ingredients (API), the efficiency of crystal formation is crucial for quality. The properties of the crystals determine the quality of the release of the active ingredient.
Efficient temperature control of the mixture is only possible with a large specific heat exchange surface. There must be no temperature gradients in the mixture. This enables uniform nucleation.
Regardless of the speed of the mixing tool, the ideal mixing quality must always be present: during the synthesis reaction as well as during crystallisation. Homogeneity, solubility, bioavailability and stability of the active ingredient are influenced by this.
Well-known chemically synthesised pharmaceutical active ingredients include paracetamol, ibuprofen, atrovistan, amoxicillin, metforin, omeprazole, aspirin and hydrochlorothiazide.
There are also active pharmaceutical ingredients that are produced biologically in the first part and synthetically in the second: for example, amoxillin, vitamin C, stanins, steroid hormones or the peptide hormone human insulin.
Hygiene, complete discharge, trials in the pilot plant
The risk of contamination is lower the better the mixers / fermenters / synthesis reactors vacuum mixing dryers are able to discharge themselves completely. This can be difficult with powders, suspensions or highly viscous products.
Over the course of 40 years, amixon® has developed and tested special solutions. In our technical centre in Paderborn, we have various systems that we would be happy to use for tests with your original product. We look forward to your visit and assure you of a high level of knowledge gain.
Modern materials with customised properties
Only materials that are optimally suited make it possible to carry out high-risk chemical processes. Four factors are crucial here:
- Resistance to corrosive attacks by alkalis and acids
- Resistance to deformation
- Resistance to cracking and fatigue
- Excellent weldability
- Excellent grindability
In addition to the classic austenitic stainless steels 1.4301 (grade 304), 1.4571 (grade 316), 1.4404 (grade 316L), the duplex stainless steels 1.4462 and super duplex stainless steels, e.g. 1.4410, are becoming established.
Super duplex stainless steels are highly corrosion resistant and have significantly better strength properties than austenitic stainless steels. They reduce the occurrence of stress corrosion cracking. Unlike austenitic stainless steels, duplex steels are less prone to pitting. The following applies to thick-walled sheets/profiles: in contrast to austenitic materials, these must be preheated before welding.
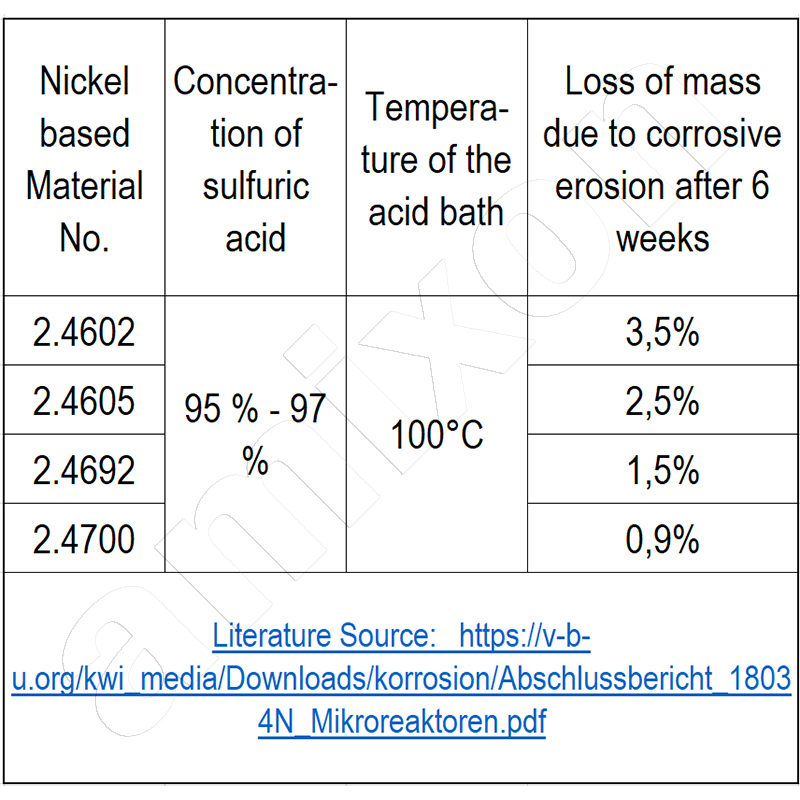
In terms of corrosion resistance and strength, Hastelloy/Alloy materials have the best properties. Unfortunately, they are also the most expensive. These materials are difficult to process and weld. amixon® has many qualifications and can weld these materials with robots.
Pure nickel materials are suitable for nitric and sulfuric acid, but are rarely used because of their lower strength and high price. Very high costs arise when apparatus or components have to be made of titanium alloys (chloride-containing acids) or tantalum alloys (nitric acid). The latter are relatively soft and unsuitable for abrasive stress.
Explosive and roll cladding
Depending on the cause of corrosion, a distinction is made between surface corrosion, pitting corrosion, crevice corrosion, contact corrosion, intergranular corrosion and stress corrosion cracking. This sometimes makes it difficult to define a suitable construction material.
In particular, two or three materials are combined in large pressure-bearing apparatus. The part that comes into contact with the product is particularly corrosion-resistant. The outer supporting structures, on the other hand, are made of less expensive materials. This approach makes sense and helps to save costs. However, it is important that the combined materials can be welded together. It is equally important that the materials have the same elasticity and thermal expansion coefficients.
amixon® has extensive experience, particularly in the welding of clad materials.
The type of material connection shown on the right is referred to as cold press welding or cold welding. This test strip was cut out of a clad rolled sheet and bent.
Solvents should be recovered
Complex synthesis steps require the use of specially adapted solvents. Solvents are expensive, volatile and hazardous to health. They are difficult to produce in the required purity. This makes them even more expensive. Examples are dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), hexafluoroisopropanol (HFIP), perfluorinated solvents (e.g. perfluorheptane), dichloromethane (methylene chloride) or acetonitrile.
The solvents should not be incinerated after use, but recovered and purified. After use, solvents are often contaminated with dissolved solids. This prohibits the use of classic rectification columns. amixon® has developed special evaporatorswith which solvent recovery works well even when concentrated solutions or suspensions are involved. The amixon® test systems are also available for trials in this regard.
History of pharmacology
Since time immemorial, people have been searching for healing methods. Initially, this was done with natural active ingredients from herbs, fruits, leaves, minerals, etc. Later, fats and oils were used as carriers for healing substances.
Even in ancient times, highly effective substances were isolated: olive oil, opium from the juice of the poppy plant, salicin from willow bark, quinine against malaria, aloe vera for burns, garlic for infections. .... thyme, myrtle, fennel, honey, leeches, sulfur, salt, alcohol, vinegar, natural asphalt/bitumen, .... Soap was invented around 2000 BC and was used as a preservative and carrier for healing substances.
From the Middle Ages to modern times, little new knowledge was gained in Europe. Medical theories were based on the teachings of antiquity. Arabic, Persian, Greek and Roman traditions were translated and disseminated in the monasteries.
Pharmacology in the early days
Major leaps in knowledge in organic chemistry occurred at the beginning of industrialisation. In 1804, the pharmacist Friedrich Sertürner isolated morphine from opium. Alder Wright discovered diacetylmorphine (heroin) in 1874. Felix Hoffmann discovered aspirin in 1897. Justus von Liebig discovered the synthetic sedative chloral hydrate in 1832. Adolf von Baeyer discovered psychotropic drugs and barbiturates in 1864. Paul Ehrlich and Sahasaburo Hata discovered the antibiotic salvarsan (arsphenamine) in 1909. The Japanese scientist Jokichi Takamine discovered adrenaline in 1901.
Around 1900, engineers developed what we today call chemical apparatus engineering. They developed corrosion-resistant materials with high strength, large stirrers, large pressure-bearing reaction vessels, pressure-resistant seals for mixer shafts, heat exchanger systems, heating and cooling units, distillation ation columns, rectification columns, centrifuges, chamber filter presses, vacuum freeze dryers, roller dryers, spray dryers, fluidised bed apparatus and ring layer agglomerators.
The combination of a wide range of process engineering equipment enables the large-scale production and formulation of synthetic active ingredients.
Wherever possible, attempts are now being made to produce medical active ingredients as gently as possible in bioreactors. In this respect, two lines of development will continue to develop side by side: biotechnological and chemical material conversion. Both can complement and benefit from each other, since sophisticated active ingredients are synthesised in several steps.
Galenics
Galenics is the branch of pharmaceutical science that deals with the preparation of active ingredients so that they are safe and easy for patients to use. The wide range of dosage forms is a central topic in galenics. These include
- Solids: tablets, capsules, powders
- Liquids: syrups, drops, solutions
- Semi-solid forms: creams, gels, ointments
- Inhalants**: sprays, aerosols
- Transdermal systems Plasters
- Parenterals Injection and infusion solutions
Highly pure active ingredients usually have to be dispersed in excipients. For example, a few micrograms of a hormonal active ingredient are distributed in a 500 mg tablet. The powdered active ingredient is then mixed into a carrier substance at a ratio of 1:1000 or even 1: 5000. amixon® is creating a separate blog post on this topic that will be available soon.
© Copyright by amixon GmbH