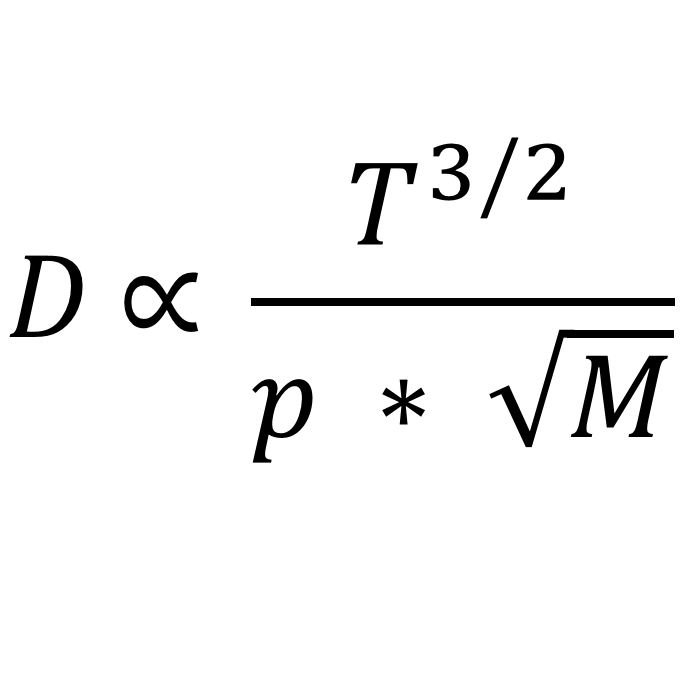
Diffusion coefficient
The diffusion coefficient quantitatively describes the speed at which molecules, atoms or ions are transported in a medium by diffusion. It can only be calculated approximately.
- D: diffusion coefficient (m²/s)
- T: absolute temperature (K)
- p: pressure (Pa)
- M: molar mass of the diffusing substance (kg/mol)
This equation shows that higher temperatures accelerate diffusion, while higher pressure slows it down.
It is also colloquially referred to as the diffusion constant and is often used synonymously in practice. In heterogeneous solid systems, such as those found in powder mixtures, the effective diffusion coefficient can be influenced by the macroscopic structure, porosity and moisture content.
Diffusion is driven by differences in concentration. Molecules, atoms and ions migrate from higher to lower concentrations. The higher the temperature, the faster this happens in the gas space.
Atomic diffusion plays a role in powder metallurgy and engineering ceramics, for example, when dissimilar microparticles are compacted and sintered under high pressure.
amixon mixers can homogenise such metallic and nanoceramic material systems ideally and precisely.
